This article based on the ICH Q1A guideline about bracketing and matrixing to stability studies of pharmaceutical products.
Bracketing is the design of a stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time points as in a full design.
Matrixing is the design of a stability schedule such that a selected subset of the total number of possible samples for all factor combinations would be tested at a specified time point.
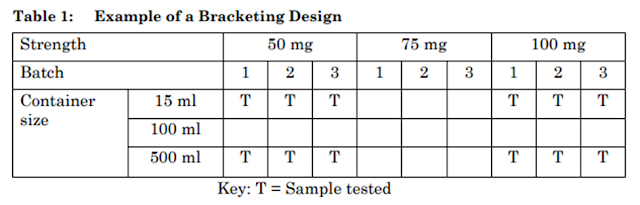
Example of a Bracketing Design
An example of a bracketing design is given in below Table-1. This example is based on a product available in three strengths and three container sizes. In this example, it should be demonstrated that the 15 ml and 500 ml high-density polyethylene container sizes truly represent the extremes. The batches for each selected combination should be tested at each time point as in a full design.
Examples of Matrixing Designs
Examples of matrixing designs on time points for a product in two strengths (S1 and S2) are shown in Table 2. The terms “one-half reduction” and “one-third reduction” refer to the reduction strategy initially applied to the full study design. For example, a “one-half reduction” initially eliminates one in every two time points from the full study design and a “one-third reduction” initially removes one in every three.


Post a Comment